
The rest and the majority of the atom is made up of space.
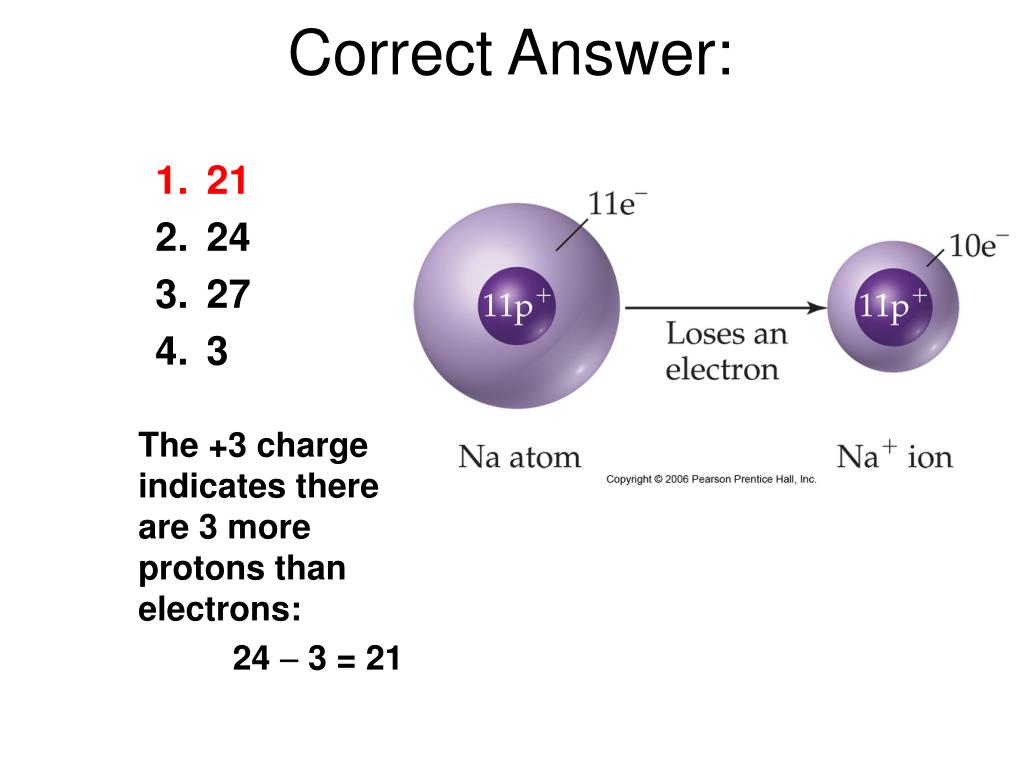
Electrons have virtually no mass their mass is often taken as zero or 1/ 2000. Electrons are negatively charged while protons are positively charged and they both exert. When the closest shell is full, the electrons will start filling the next shell out for example, if the first shell is full, the electrons will start filling the second shell. Because positive ions have stronger pulls on their electrons. An electron will always fill the closest shell to the nucleus. The first shell holds a maximum of 2 electrons, the second and third shells hold a maximum of 8 electrons. There are many electron shells and each of the shells can only hold a certain number of electrons. These shells are referred to as electron shells or energy levels. An atom that has as many protons as electrons is neutrally-charged.

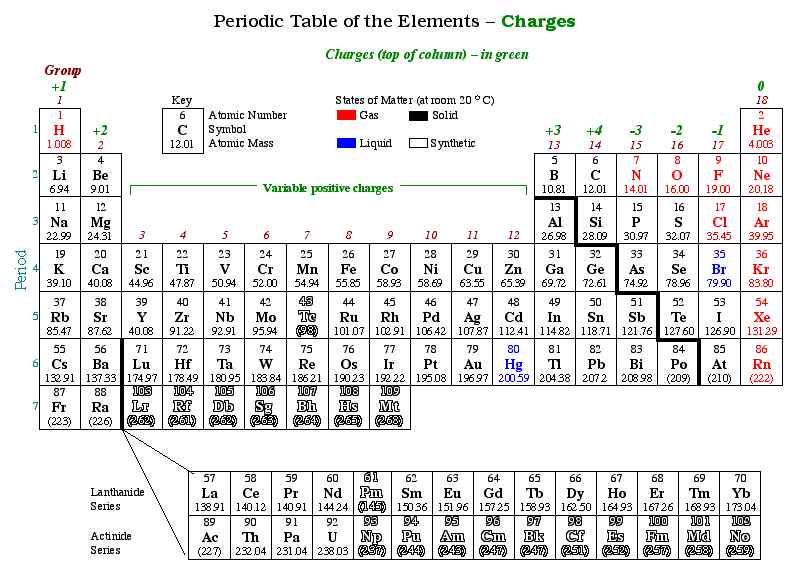
Almost all of the mass in an atom is found in the nucleus and this is because all of the protons and neutrons are found in the nucleus.Įlectrons move around the nucleus in shells. When an atom has more electrons than protons, it is negatively-charged. The nucleus of an atom is positively charged because the protons are found in the nucleus and they have a positive charge (neutrons are also found in the nucleus, but they have no charge and therefore no effect on the charge of the nucleus). The nucleus is tiny in comparison to the size of the rest of the atom the radius of the nucleus is about 10,000 times smaller than the radius of the atom (the radius of an atom is about 1 x 10 -14 m). Protons and neutrons are found in the nucleus of an atom (the centre of an atom).


 0 kommentar(er)
0 kommentar(er)
